Featured on the cover of the April 19, 2010 issue of the International Edition of the journal Angewandte Chemie, this molecule may be useful as a laboratory tool for controlling tiny reactions in the test tube, and it has potential to be developed as the basis of a new technology that could sensitively detect metals, toxins, and other pollutants in the air, water, or soil.
The molecule is named "ouroborand" after the mythical Ouroboros ("tail-eater" in Greek) â€" a lizard-like creature that swallows itself head-to-tail.
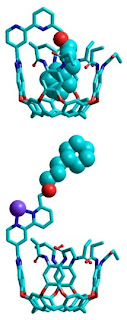
In the Scripps Research laboratory where it was invented, the ouroborand molecule alternatively swallows or coughs out its tailâ€"like a switch that goes on or off as it senses metals. (Illustrated by Fabien Durola of the Rebek lab; armadillo picture on the News&Views homepage courtesy of P. le F. N. Mouton.) In mythology, the cyclic Ouroboros is always depicted with its tail in its mouth and this is usually taken as a symbol of eternity. In the Scripps Research laboratory where it was invented, the ouroborand molecule alternatively swallows or coughs out its tailâ€"like a switch that goes on or off as it senses metals.
This switching is possible because the molecule has a cup-like head at one end and a tail at the other. The tail can curl around and plug the cup â€" just like the lizard swallowing its own tail.
"When no metals are present, the molecule's tail is held within its cavity at the other end," says Julius Rebek, who is the director of the Skaggs Institute for Chemical Biology at Scripps Research.
In the presence of zinc or other metal ions, the part of the molecule that links the head and the tail curls around the metal ions and pulls the head and the tail apart, springing the molecule open, says Rebek. Remove the metal, and the tail will move again to plug the other end of the molecule.
When Rebek and his postdoctoral fellow Fabien Durola synthesized the molecule and decided to name it ouroborand, they made a return of sorts to the dreams of chemists and alchemists long ago. In Medieval times and through the Renaissance, the mythical Ouroboros was a symbol used in alchemy, the ancient practice that was a forerunner to modern chemistry.
The spirit of this symbol later briefly carried over into modern chemistry as well. More than a century ago, the famous German chemist August Kekule had a dream about a serpent with its tail in its mouth, and this inspired him to propose the correct, circular structure of the compound benzene, a commonly used industrial solvent.
This month Rebek is presenting a lecture at the Kekule Institute at the University of Bonn in Germany, where he will formally unveil the new Ouroborand molecule â€" in all its tail-eating glory.The Angewandte Chemie article, "The Ouroborand: A Cavitand with a Coordination-Driven Switching Device" is authored by Fabien Durola and Julius Rebek and is available online: DOI: 10.1002/anie.200906753, see www3.interscience.wiley.com/journal/123345195/abstract
This work was funded by the National Institutes of Health and the Skaggs Institute for Chemical Biology, and supported by a fellowship from the French Ministry of Foreign Affairs.
Contact: Keith McKeown kmckeown@scripps.edu 858-784-8134 Scripps Research Institute
