The collaboration of researchers of the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) and of the Department of Chemistry, Wichita State University, Wichita, KS, has resulted in fabrication of a polymer trap for nicotine. Bearing molecular pincers, the polymer effectively captures nicotine molecules and its analogues, and can also release them in a controlled way. The compound will be used in reusable chemical sensors for determination of nicotine for industrial and biomedical purposes as well as in patches for smokers to evenly release nicotine to the body for a prolong time.
"The first nicotine trap has been synthesized by our US partner, Prof. Francis D'Souza, several years ago. It was a sort of molecular pincers, molecules that freely move in solution and form complexes with nicotine therein. Recently, our US-Polish team has been able to fix the pincers inside a polymer. The substance is solid, and that's why we could use it to construct chemosensors", says Prof. WÅ‚odzimierz Kutner from IPC PAS.
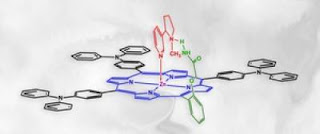
Caption: Polymer nicotine trap is composed of a porphyrin derivative (black), in which amide pincers (green) are attached to the zinc (violet) containing macrocycle (blue). The nicotine molecule is shown in red.
Credit: IPC PAS/Tentaris/ACh. Usage Restrictions: With credit.
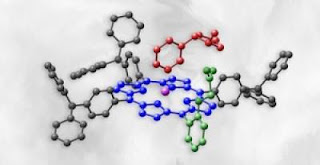
Caption: Polymer nicotine trap is composed of a porphyrin derivative (black), in which amide pincers (green) are attached to the zinc (violet) containing macrocycle (blue). The nicotine molecule is shown in red.
Credit: IPC PAS/Tentaris/ACh. Usage Restrictions: With credit.The core of the polymer nicotine trap, which has been recently filed for a patent, is a metalloporphyrin derivative, a substance present, i.a., in human blood. The molecule contains a ring (a macrocycle) with a centrally located zinc atom and amide pincers attached to this ring. Nicotine binds to this polymer with its two nitrogen atoms: one binds to the zinc atom, whereas the other to the pincers. "It is due to specific two-point binding that we are surer that the captured molecule is nicotine", stresses Dr. Krzysztof Noworyta from IPC PAS, adding that in one of the devised polymers the pincers are located on both sides of the zinc containing-ring plane. "Such a design clearly increases the efficiency of nicotine trapping", says Dr. Noworyta.
Beside nicotine, the polymer captures also a cotinine alkaloid produced in the metabolism of nicotine and other alkaloids often accompanying nicotine, e.g., myosmine. Polymer binding to nicotine is durable but reversible. It is the property why the new chemosensors for determination of nicotine and its analogues can be used repeatedly.
Nicotine is detected by means of a piezoelectric resonator coated by electropolymerization with a submicrometer thick polymer film. The captured nicotine increases the mass of the film resulting in a decrease in the resonant frequency of the resonator that is easy to measure.
"It can be said that we are weighing a film of our polymer all throughout the experiment. Because we know the initial polymer mass and we know that the polymer selectively captures nicotine and its analogues, an increased mass of the film means that these compounds are present in solution", explains Dr. Noworyta.
Quartz acoustic bulk wave resonators used in experiments with the new polymer allow determining nicotine in solutions. In the near future, the researchers from IPC PAS plan to establish collaboration with manufacturers of surface acoustic wave resonators. These resonators oscillate at significantly higher frequencies, thus being more sensitive, and after coating with the nicotine capturing polymer film could detect nicotine also in gases.
In the method described herein, the detection and determination of nicotine do not need to be confined to weighing. Because nicotine is electroactive, the researchers from IPC PAS are going to measure oxidation current of nicotine trapped in the polymer in parallel with the resonant frequency measurement. Simultaneous measurement with these two methods will increase the detection reliability.
The polymer with pincers for nicotine can be used, among others, in chemosensors devised to analyze nicotine content in tobacco leaves and in biomedical studies to determine nicotine metabolites in patients' body fluids. Another potential application is nicotine patches to help quit smoking. The new polymer could be used for prolong and smooth release of nicotine.
On the Polish side, the research described herein has been performed within the project "Quantum semiconductor nanostructures for applications in biology and medicine". The project, funded in 85% from the European Regional Development Fund, has been awarded to seven Polish research institutions for construction of prototype diagnostic devices on semiconductor substrates, designed for applications in biology, medicine, and environment protection as well as for the development of fundamentals of materials technologies for sensor applications and molecular diagnostics devices. Almost 50 researchers from IPC PAS participate in the project implementation.
###
The Institute of Physical Chemistry of the Polish Academy of Sciences (www.ichf.edu.pl/) was established in 1955 as one of the first chemical institutes of the PAS. The Institute's scientific profile is strongly related to the newest global trends in the development of physical chemistry and chemical physics. Scientific research is conducted in nine scientific departments. CHEMIPAN R&D Laboratories, operating as part of the Institute, implement, produce and commercialise specialist chemicals to be used, in particular, in agriculture and pharmaceutical industry. The Institute publishes approximately 200 original research papers annually.
CONTACTS:
Dr. Eng. Krzysztof Noworyta Institute of Physical Chemistry of the Polish Academy of Sciences
tel.: +48 22 3433217 e-mail: know@ichf.edu.pl
Prof. WÅ‚odzimierz Kutner Institute of Physical Chemistry of the Polish Academy of Sciences tel.: +48 22 3433217 e-mail: wkutner@ichf.edu.pl
Prof. Francis D'Souza Department of Chemistry, University of North Texas, 1155, Union Circle, #305070 Denton, TX 76203-5017 tel: (+) 940 369 8832 e-mail: francis.dsouza@unt.edu
Contact: Antoni Szafranski press@ichf.edu.pl WEB: Institute of Physical Chemistry of the Polish Academy of Sciences
